Molecular Neurophysiology
Stories
PhD available
Ph.D. POSITION to study the MECHANISMS of NEURON-GLIA COMMUNICATION at the UNIVERSITY OF SAARLAND (Homburg/Saar) Germany.
A Ph.D. position is available to study the molecular mechanisms that mediate neuron-glia communication. The challenge is to delineate molecular events that couple Ca2+ signaling to fusion of secretory vesicles with the plasma membrane. Our research addresses these tasks using state-of-the-art electro-physiological methods (carbon fiber amperometry and membrane capacitance measurements), imaging techniques (Fura II measurements, photolytic ‘uncaging’ of Ca2+, confocal microscopy, structured illumination and STED microscopy) and mice strains that are genetically deficient for synaptic proteins in order to discover, characterize, and disrupt protein-protein interactions that operate in excitation-secretion coupling.
The work is directly supported by technical personnel and applicants are given the opportunity to extend their research interest internationally through project–oriented collaborations. Applicants with experience in electrophysiology, molecular biology, fluorescence spectroscopy are especially encouraged to apply.
Please send your application including curriculum vitae, list of publications and a brief statement of research interest as one pdf file to Prof. Dr. Dieter Bruns.
Selected readings:
Kollewe A, Schwarz Y, Oleinikov K, Raza A, Haupt A, Wartenberg P, Wyatt A, Boehm U, Ectors F, Bildl W, Zolles G, Schulte U, Bruns D, Flockerzi V, Fakler B.
Subunit composition, molecular environment, and activation of native TRPC channels encoded by their interactomes. Neuron. 2022
Dhara M, Mantero Martinez M, Makke M, Schwarz Y, Mohrmann R, Bruns D. Synergistic actions of v-SNARE transmembrane domains and membrane-curvature modifying lipids in neurotransmitter release. Elife. 2020
Schwarz Y, Oleinikov K, Schindeldecker B, Wyatt A, Weißgerber P, Flockerzi V, Boehm U, Freichel M, Bruns D. TRPC channels regulate Ca2+-signaling and short-term plasticity of fast glutamatergic synapses. PLoS Biol. 2019,17.
Schwarz Y, Zhao N, Kirchhoff F, Bruns D. Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci. 2017,20, see Faculty of 1000.
DPG – Paper of the Month Dezember 2022
DPG – Paper of the Month Dezember 2022
Subunit composition, molecular environment, and activation of native TRPC channels encoded by their interactomes
Kollewe A, Schwarz Y, Oleinikov K, Raza A, Haupt A, Wartenberg P, Wyatt A, Boehm U, Ectors F, Bildl W, Zolles G, Schulte U, Bruns D, Flockerzi V & Fakler B.
TRP (Transient Rezeptor Potential) Kanäle wurden zuerst im Auge der Fruchtfliege Drosophila melanogaster entdeckt und 2021 mit dem Nobelpreis für Medizin ausgezeichnet (David Julius und Ardem Patapoutian). Von allen beschriebenen TRP Kanal Unterfamilien der Säuger, zeigen TRPC Kanäle die größte Ähnlichkeit zu den ursprünglich in der Fruchtfliege identifizierten. Sie werden in vielen Organen exprimiert und spielen unter anderem bei der Informations¬weiter¬gabe zwischen Nervenzellen eine wichtige Rolle. Ihre Aktivierung und Regulation sind trotz ihrer Bedeutung jedoch nur teilweise geklärt. Wissenschaftlern der Universität Freiburg um Astrid Kollewe und Bernd Fakler ist es in Zusammenarbeit mit Kollegen Yvonne Schwarz, Dieter Bruns und Veit Flockerzi von der Universität des Saarlands in Homburg gelungen, den molekularen Aufbau nativer TRPC Kanäle im Gehirn von Mäusen und ihren Aktivierungs¬mechanismus zu entschlüsseln. Dabei zeigte sich, dass schon beim Zusammenbau der TRPC Proteine zu Kanalporen - im Säuger besteht die Familie aus sieben Mitgliedern (TRPC1-TRPC7) - nicht alle Kombinationsmöglichkeiten genutzt werden, die experimentell möglich sind. Mittels hochauflösender Proteomanalyse-Techniken identifizierten die Forscher außerdem 15 weitere Proteine, die im Maushirn mit TRPC1/C4/C5 Kanälen interagieren und deren Funktionsweise mutmaßlich beeinflussen, zum Teil aber bisher noch gar nicht mit TRPC Kanälen in Verbindung gebracht worden waren.
Analyse eines der Interaktionspartner, des metabotropen Glutamatrezeptors mGluR1, hat den Wissenschaftlern wichtige Informationen über die Aktivierung der TRPC Kanäle geliefert: Glutamatbindung an mGluR1 setzt in der Zelle eine Signalkette in Gang, in deren Verlauf Ca2+ aus intrazellulären Kompartimenten ins Zytosol freigesetzt wird. Geschieht das in direkter Nachbarschaft zum TRPC Kanal, reicht das ausgeschüttet Ca2+ aus, um den Kanal zu aktivieren. Auch, wenn dieser Mechanismus theoretisch ohne eine direkte Interaktion zwischen Rezeptor und Kanal auskommen könnte, dient die von der Natur gewählte Komplexbildung wohl dazu, die Signalweiterleitung zuverlässiger zu machen, die Aktivierung räumlich zu begrenzen, und die Signalweitergabe zu beschleunigen. Offen ist, ob die hier entdeckte direkte Bindung zwischen einem Rezeptor und dem sekundären Effektor beispielgebend für ein häufiger genutztes Prinzip in Signalketten ist.
Neuron. 2022 https://doi.org/10.1016/j.neuron.2022.09.029.
Saarlands beste Biologie-Laborantin
Herzlichen Glückwunsch!
Wir gratulieren unserer Auszubildenden Charlotte Geißel zu ihrem hervorragenen Abschluss!

Presseartikel 24. Okt 2017


Dienstag, 24. Oktober 2017
Physiologen entdecken bisher unbekannten Einfluss auf die Signalübertragung im Gehirn
Forscher der Universität des Saarlandes um Professor Dieter Bruns konnten erstmals nachweisen, dass Astrozyten, die zu den wichtigsten Bestandteilen des Gehirns zählen, einen aktiven Einfluss bei der Signalübertragung im Gehirn haben. Astrozyten gehören zu der Gruppe der Gliazellen und galten bis vor nicht allzu langer Zeit noch als Zellen, die lediglich dazu dienen, dem Gehirn Stabilität zu verleihen, damit die Nervenzellen ihre Arbeit verrichten können: Signale von Zelle zu Zelle zu schicken. Die Stärke der Signale wird jedoch aktiv von den Astrozyten reguliert, wie Dieter Bruns und seine Kollegen nun zeigen konnten. Die Erkenntnisse könnten Grundlage neuartiger Therapieansätze für neurologische Erkrankungen wie beispielsweise Epilepsie sein. Ihre Studie erscheint morgen in der November-Ausgabe der renommierten Fachzeitschrift „Nature Neuroscience“.
Für Epileptiker sind die Anfälle eine schwere Belastung: Plötzliche, unkontrollierte Übererregungen der Nervenzellen führen zu Anfällen, mit denen Bewusstlosigkeit und schwere Krämpfe einhergehen können und in deren Folge Nervenzellen absterben. Physiologen der Universität des Saarlandes um Professor Dieter Bruns haben nun einen Mechanismus entdeckt, der eventuell eine Grundlage für neue Therapieformen neurologischer Krankheiten wie der Epilepsie sein könnte.
Im wissenschaftlichen Fokus der saarländischen Forscher stehen die Astrozyten, eine Form so genannter Gliazellen, die etwa die Hälfte der Gehirnmasse ausmachen. Noch gegen Ende des 19. Jahrhunderts bezeichnete der Berliner Pathologe Rudolf Virchow die Gesamtheit der Gliazellen als „Nervenkitt“. In der Tat galten Gliazellen lange nur als Stützzellen im Gehirn, die den Nervenzellen bei der Weiterleitung elektrischer Erregungen helfen, jedoch keinen aktiven Einfluss auf die neuronale Signalverarbeitung haben. Dabei löst ein elektrisches Signal an den Kontaktstellen der Nervenzellen, den Synapsen, die Ausschüttung chemischer Botenstoffe aus, die von der nächsten Nervenzelle gebunden werden, um wiederum ein elektrisches Signal zu erzeugen und weiterzuleiten. Diese Vorgänge laufen binnen weniger Tausendstel Sekunden ab. Veränderungen in der synaptischen Übertragung bilden die wichtigste Grundlage für Lernen und Gedächtnisleistungen unseres Gehirns.
Das Forscherteam um Professor Bruns konnte in seiner Studie nun erstmals zeigen, dass Astrozyten unterschiedliche Botenstoffe freisetzen, um die neuronale Signalübertragung zu verstärken oder abzuschwächen. Durch ihre detaillierten Analysen konnten die Wissenschaftler mehrere Mechanismen entschlüsseln, die Astrozyten zur Freisetzung von Botenstoffen befähigen.
Die Befunde liefern dadurch nicht nur neue Einblicke in die außergewöhnlich komplexe Signalverarbeitung in unserem Gehirn, sondern sind auch von Bedeutung für neurologische Erkrankungen, wie zum Beispiel der Epilepsie. Die Astrozyten können hier als Mediatoren wirken, die epilepsietypische Übererregungen mit ihren hemmenden Botenstoffen dämpfen. Die Erkenntnisse der Arbeitsgruppe eröffnen somit auch die Möglichkeit, Erkrankungen des mit neuen Therapieansätzen zu behandeln.
Die Studie von Schwarz et al. „Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways“ erscheint am 25. Oktober 2017 in der Fachzeitschrift „Nature Neuroscience“ (doi:10.1038/NN.4647, Link).
Weitere Informationen:
Prof. Dr. Dieter Bruns
Tel.: (06841) 1616494
E-Mail: Dieter.Bruns(at)uks.eu
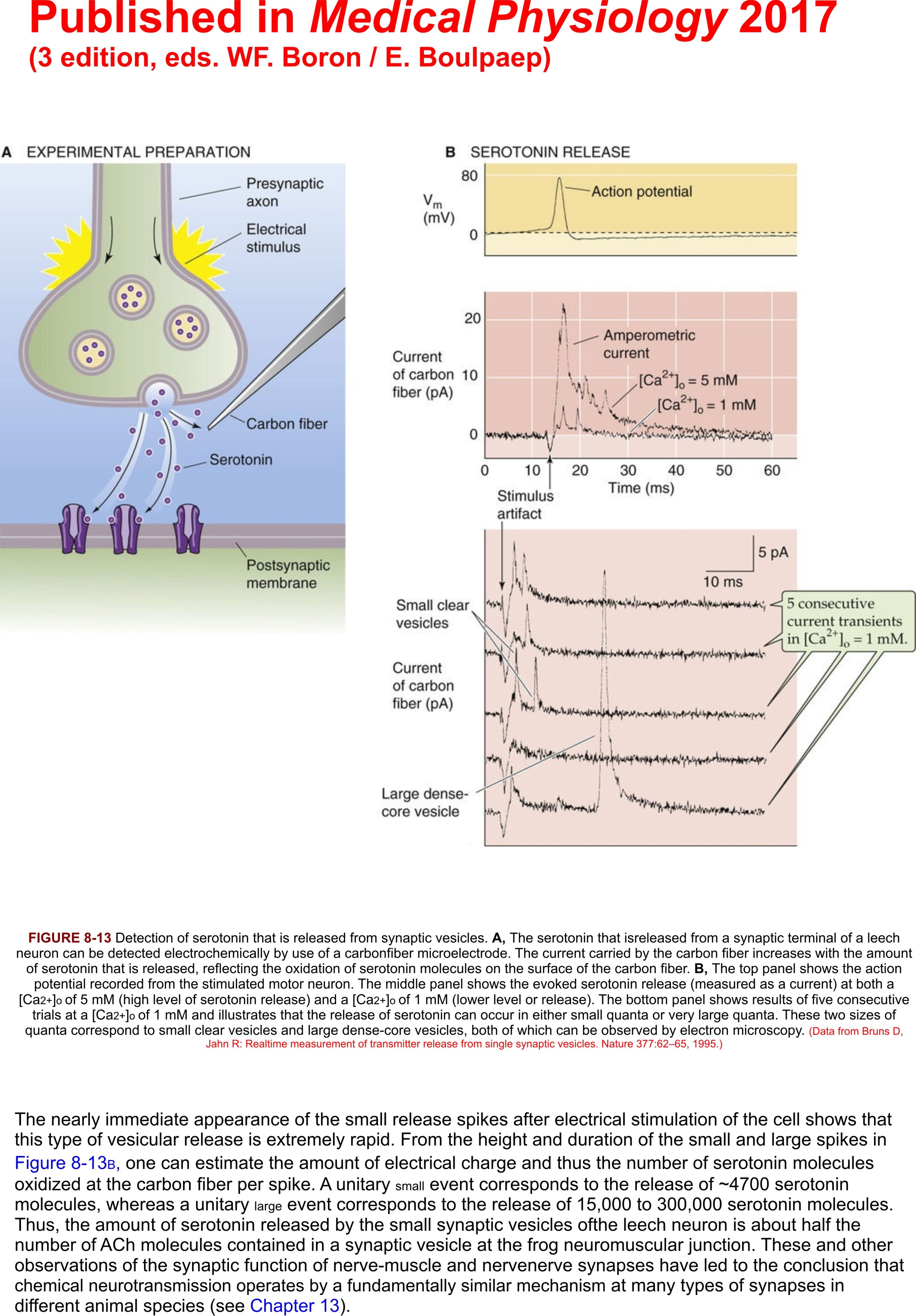
Presseartikel Medical Physiology 2017
Presseartikel FAZ 4. Okt 1995
Ph.D Positons AVAILABLE
to study
MOLECULAR MECHANISMS MEDIATING NEUROTRANSMITTER RELEASE
at the UNIVERSITY OF SAARLAND
(Homburg/Saar) Germany
Ph.D. positions are available to elucidate the molecular mechanisms that mediate neuronal exocytosis. Our goal is to define the molecular events that couple Ca2+ signaling to the release of neurotransmitter. We are addressing this problem with state-of-the-art molecular (knock-out mice),biochemical, electrophysiological and fluorescence microscopy techniques (e.g. carbon fiber amperometry, membrane capacitance measurements, Ca2+-imaging, SIM and STED microscopy). The work is directly supported by technical personnel and applicants are given the opportunity to extend their research interest internationally through project–oriented collaborations with groups in the USA. Applicants with a physical, biochemical or biological background are encouraged to apply.
Please send your curriculum vitae and a brief statement of research interest to: Prof. Dr. Dieter Bruns, Dept. of Physiology, Center of Integrated Physiology and Molecular Medicine, University of Saarland, 66421 Homburg, Building 48, Germany, Email:
Exemplary publications:
Schwarz Y, Oleinikov K, Schindeldecker B, Wyatt A, Weißgerber P, Flockerzi V, Boehm U, Freichel M, Bruns D (2019). TRPC channels regulate Ca2+-signaling and short-term plasticity of fast glutamatergic synapses. PLOS Biol., doi: 10.1371/journal.pbio.3000445
Makke M, Mantero Martinez M, Gaya S, Schwarz Y, Frisch W, Silva-Bermudez L, Jung M2, Mohrmann R, Dhara M, Bruns D (2018). A mechanism for exocytotic arrest by the Complexin C-terminus. Elife. doi: 10.7554/eLife.38981.
Dhara M, Mohrmann R, Bruns D (2018). v-SNARE function in chromaffin cells. Pflugers Arch., 470:169-180.
Schwarz Y, Zhao N, Kirchhoff F, Bruns D. (2017). Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci.20:1529-1539.
neue Publikation
PLoS Biol. 2019 Sep 19;17(9)
TRPC channels regulate Ca2+-signaling and short-term plasticity of fast glutamatergic synapses.
Schwarz Y, Oleinikov K, Schindeldecker B, Wyatt A, Weißgerber P, Flockerzi V, Boehm U, Freichel M, Bruns D.
Transient receptor potential (TRP) proteins form Ca2+-permeable, nonselective cation channels, but their role in neuronal Ca2+ homeostasis is elusive. In the present paper, we show that TRPC channels potently regulate synaptic plasticity by changing the presynaptic Ca2+-homeostasis of hippocampal neurons. Specifically, loss of TRPC1/C4/C5 channels decreases basal-evoked secretion, decreases the pool size of readily releasable vesicles, and accelerates synaptic depression during high-frequency stimulation (HFS). In contrast, primary TRPC5 channel-expressing neurons, identified by a novel TRPC5-τ-green fluorescent protein (τGFP) knockin mouse line, show strong short-term enhancement (STE) of synaptic signaling during HFS, indicating a key role of TRPC5 in short-term plasticity. Lentiviral expression of either TRPC1 or TRPC5 turns classic synaptic depression of wild-type neurons into STE, demonstrating that TRPCs are instrumental in regulating synaptic plasticity. Presynaptic Ca2+ imaging shows that TRPC activity strongly boosts synaptic Ca2+ dynamics, showing that TRPC channels provide an additional presynaptic Ca2+ entry pathway, which efficiently regulates synaptic strength and plasticity.
DOI
Publikations -News (2020)
Elife. 2020 May 11;9.
Synergistic actions of v-SNARE transmembrane domains and membrane-curvature modifying lipids in neurotransmitter release
Dhara M1, Mantero Martinez M1, Makke M1, Schwarz Y1, Mohrmann R2, Bruns D1.
1 Institute for Physiology, Center of Integrative Physiology and Molecular Medicine, University of Saarland, Homburg, Germany.
2 Institute of Physiology, Otto-von-Guericke University, Magdeburg, Germany.
Vesicle fusion is mediated by assembly of SNARE proteins between opposing membranes. While previous work suggested an active role of SNARE transmembrane domains (TMDs) in promoting membrane merger (Dhara et al., 2016), the underlying mechanism remained elusive. Here, we show that naturally-occurring v-SNARE TMD variants differentially regulate fusion pore dynamics in mouse chromaffin cells, indicating TMD flexibility as a mechanistic determinant that facilitates transmitter release from differentially-sized vesicles. Membrane curvature-promoting phospholipids like lysophosphatidylcholine or oleic acid profoundly alter pore expansion and fully rescue the decelerated fusion kinetics of TMD-rigidifying VAMP2 mutants. Thus, v-SNARE TMDs and phospholipids cooperate in supporting membrane curvature at the fusion pore neck. Oppositely, slowing of pore kinetics by the SNARE-regulator complexin-2 withstands the curvature-driven speeding of fusion, indicating that pore evolution is tightly coupled to progressive SNARE complex formation. Collectively, TMD-mediated support of membrane curvature and SNARE force-generated membrane bending promote fusion pore formation and expansion.
Publikations -News
A mechanism for exocytotic arrest by the Complexin C-terminus.
ComplexinII (CpxII) inhibits non-synchronized vesicle fusion, but the underlying mechanisms have remained unclear. Here, we provide evidence that the far C-terminal domain (CTD) of CpxII interferes with SNARE assembly, thereby arresting tonic exocytosis. Acute infusion of a CTD-derived peptide into mouse chromaffin cells enhances synchronous release by diminishing premature vesicle fusion like full-length CpxII, indicating a direct, inhibitory function of the CTD that sets the magnitude of the primed vesicle pool. We describe a high degree of structural similarity between the CpxII CTD and the SNAP25-SN1 domain (C-terminal half) and show that the CTD peptide lowers the rate of SDS-resistant SNARE complex formation in vitro. Moreover, corresponding CpxII:SNAP25 chimeras do restore complexin's function and even 'superclamp' tonic secretion. Collectively, these results support a so far unrecognized clamping mechanism wherein the CpxII C-terminus hinders spontaneous SNARE complex assembly, enabling the build-up of a release-ready pool of vesicles for synchronized Ca2+-triggered exocytosis.
Presseartikel SZ vom 17.01.2018
Link: Saarbrücker Zeitung
aktuelle Publikation
Nat Neurosci. 2017 Sep 25
Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways.
Schwarz Y., Zhao N., Kirchhoff F., Bruns D.

Abstract
Communication between glia cells and neurons is crucial for brain functions, but the molecular mechanisms and functional consequences of gliotransmission remain enigmatic. Here we report that astrocytes express synaptobrevin II and cellubrevin as functionally non-overlapping vesicular SNARE proteins on glutamatergic vesicles and neuropeptide Y-containing large dense-core vesicles, respectively. Using individual null-mutants for Vamp2 (synaptobrevin II) and Vamp3 (cellubrevin), as well as the corresponding compound null-mutant for genes encoding both v-SNARE proteins, we delineate previously unrecognized individual v-SNARE dependencies of astrocytic release processes and their functional impact on neuronal signaling. Specifically, we show that astroglial cellubrevin-dependent neuropeptide Y secretion diminishes synaptic signaling, while synaptobrevin II-dependent glutamate release from astrocytes enhances synaptic signaling. Our experiments thereby uncover the molecular mechanisms of two distinct v-SNARE-dependent astrocytic release pathways that oppositely control synaptic strength at presynaptic sites, elucidating new avenues of communication between astrocytes and neurons.
DOI
Publikations - Neuigkeiten
EMBO J. 2017 Sep 15
Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory.
Bröker-Lai J, Kollewe A, Schindeldecker B, Pohle J, Nguyen Chi V, Mathar I, Guzman R, Schwarz Y, Lai A, Weißgerber P, Schwegler H, Dietrich A, Both M, Sprengel R, Draguhn A, Köhr G, Fakler B, Flockerzi V, Bruns D, Freichel M.

Author information
Abstract
Canonical transient receptor potential (TRPC) channels influence various neuronal functions. Using quantitative high-resolution mass spectrometry, we demonstrate that TRPC1, TRPC4, and TRPC5 assemble into heteromultimers with each other, but not with other TRP family members in the mouse brain and hippocampus. In hippocampal neurons from Trpc1/Trpc4/Trpc5-triple-knockout (Trpc1/4/5-/-) mice, lacking any TRPC1-, TRPC4-, or TRPC5-containing channels, action potential-triggered excitatory postsynaptic currents (EPSCs) were significantly reduced, whereas frequency, amplitude, and kinetics of quantal miniature EPSC signaling remained unchanged. Likewise, evoked postsynaptic responses in hippocampal slice recordings and transient potentiation after tetanic stimulation were decreased. In vivo, Trpc1/4/5-/- mice displayed impaired cross-frequency coupling in hippocampal networks and deficits in spatial working memory, while spatial reference memory was unaltered. Trpc1/4/5-/- animals also exhibited deficiencies in adapting to a new challenge in a relearning task. Our results indicate the contribution of heteromultimeric channels from TRPC1, TRPC4, and TRPC5 subunits to the regulation of mechanisms underlying spatial working memory and flexible relearning by facilitating proper synaptic transmission in hippocampal neurons.
© 2017 The Authors.KEYWORDS:
TRPC1/C4/C5 heteromeric assembly; cross‐frequency coupling; hippocampal synaptic transmission; relearning; spatial working memory
DOI
Publikations - Neuigkeiten
v-SNARE function in chromaffin cells.
Abstract
Vesicle fusion is elementary for intracellular trafficking and release of signal molecules, thus providing the basis for diverse forms of intercellular communication like hormonal regulation or synaptic transmission. A detailed characterization of the mechanisms underlying exocytosis is key to understand how the nervous system integrates information and generates appropriate responses to stimuli. The machinery for vesicular release employs common molecular players in different model systems including neuronal and neuroendocrine cells, in particular members of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) protein family, Sec1/Munc18-like proteins, and other accessory factors. To achieve temporal precision and speed, excitable cells utilize specialized regulatory proteins like synaptotagmin and complexin, whose interplay putatively synchronizes vesicle fusion and enhances stimulus-secretion coupling. In this review, we aim to highlight recent progress and emerging views on the molecular mechanisms, by which constitutively forming SNAREpins are organized in functional, tightly regulated units for synchronized release. Specifically, we will focus on the role of vesicle associated membrane proteins, also referred to as vesicular SNAREs, in fusion and rapid cargo discharge. We will further discuss the functions of SNARE regulators during exocytosis and focus on chromaffin cell as a model system of choice that allows for detailed structure-function analyses and direct measurements of vesicle fusion under precise control of intracellular [Ca]i.
KEYWORDS:
Ca2+-triggered exocytosis; Exocytosis; Membrane fusion; SNARE proteins; SNARE regulators
Pagliarello-Studienpreis 2016 und Eduard-Martin-Preis 2017
Madhurima Dhara gewann den 2000 Euro dotierten Pagliarello - Studienpreis 2016
sowie den Dr. Eduard-Martin-Preis 2017
der Universitätsgesellschaft des Saarlandes

Sie wurde für ihre Promotionsarbeit" Role of v-SNARE transmembrane domain and of SNARE regulators in Ca 2+ -triggeres exocytosis" ausgezeichnet.
Herzlichen Glückwunsch
Molecular Neurophysiology

Univ.-Prof. Dr.
Dieter Bruns
| +49 6841/16-16494 | |
| +49 6841/16-16492 |
Labmanager
Marina Roter
Saarland University
Faculty of Medicine
Institut of Physiology
Univ.-Prof. Dr. Dieter Bruns
CIPMM | Building 48
D-66421 Homburg