Molecular Imaging
Research and Techniques/ Open Positions
M. Lauterbach | Molecular Imaging
The goal of this lab is to understand the interplay of ultrastructure and function in neurons and glia. To reach this goal, we apply and develop modern optical and electrophysiological techniques, particularly in the field of superresolution microscopy. Using an interdisciplinary approach between physics and neuroscience we bridge the gap between developing new microscopy techniques and directly applying them.
For optical techniques, we are focusing on STED microscopy (Stimulated Emission Depletion microscopy), which is an optical superresolution technique for imaging living and fixed tissue with a resolution beyond the diffraction limit. STED microscopy gives us information about the structure and structural changes. We then combine this information with functional recordings using calcium imaging and electrophysiological techniques. Additionally, we are developing new imaging approaches and are creating a microendoscopy system for the functional imaging of cellular signals.
In addition, the lab is interested in exploring the questions of structure and function in non-traditional model organisms for the purpose of identifying evolutionarily conserved mechanisms and for experimental advantages. Therefore, our main model organism is the turtle, but we also work with mice.
For the open positions, please refer to the bottom of this page.
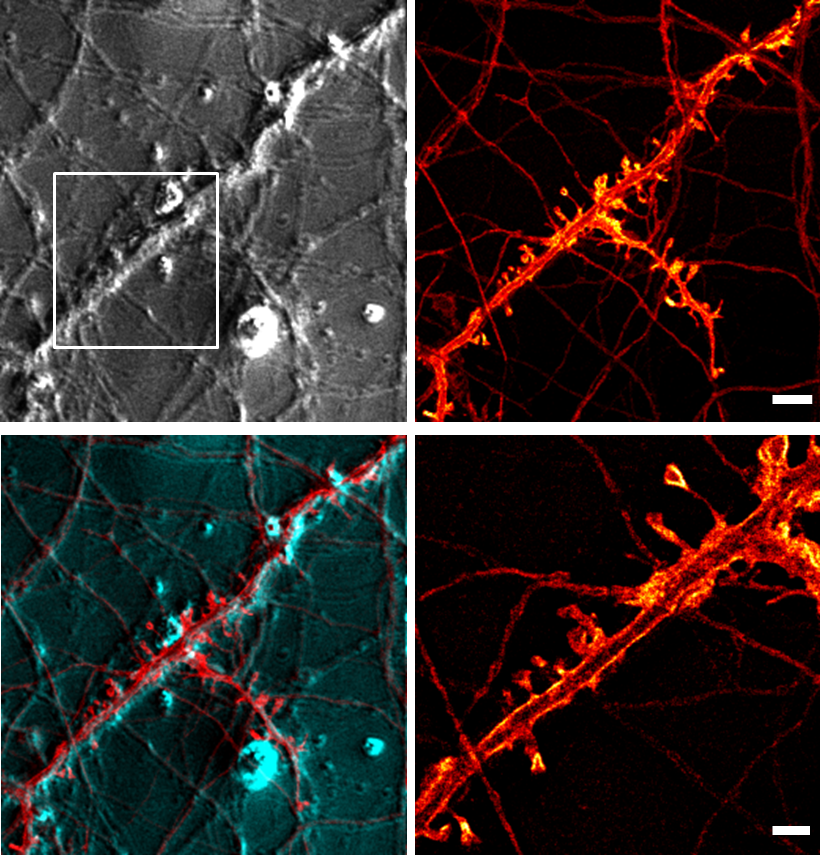 |
STED microscopy
STED microscopy is an optical so-called „superresolution“ microscopy technique that gives a higher resolution than conventional optical microscopes. This higher resolution translates into a higher useful magnification. Its invention was recognized by the Nobel Prize for Chemistry in 2014.
Calcium Imaging
Calcium is an important signaling molecule in neurons and glia. The activity of the cells is correlated with changes in intracellular calcium concentration. Indicator dyes allow converting these calcium-concentration changes into changes of fluorescence. The activity of the cells can thus be monitored by imaging fluorescence intensity. In this way, many cells in the network can be monitored simultaneously.
Microendoscopy
To enable in-vivo recording of functional signals we are developing a tiny microendoscopy system. Based on a multicore fibre bundle our system aims to resolve functional single-cell signals. With this system we are smaller than existing approaches while having multichannel functionality. The microendoscope allows us to record single-cell responses in inter-organ communication and in varying disease models.
Electrophysiology
Microelectrodes allow the recording of the membrane potential of cells. Their activity and generation of action potentials can thus be monitored with high temporal resolution.
Turtles
The evolutionary position of reptiles relative to mammals makes the reptile brain an interesting model system to study the structural and functional evolution of neuronal circuits of vertebrates. Turtles have an unfolded dorsal cortex that is divided into only three cell layers – in contrast to the mammalian cortex that has six layers. Thus, the turtle cortex is similar to the parts of the mammalian brain (hippocampus, olfactory bulb) that are considered to be evolutionarily old. Here, general – evolutionary old – principles of neuronal signal processing can probably be discovered and understood.
Transgenic Mice
In order to identify the morphology and the function of neurons in certain brain areas, we need to specifically visualize them. Taking advantage of various transgenic mice from our cooperation partners in the Department of Molecular Physiology we induce the expression of fluorescent proteins in specific cell types and brain areas.
Open Positions
|
|
Secretary / Assistant |
We look forward to receiving your online application (in a PDF file) by 07/09/2025 to
For further details see the PDF file.
We are always looking for motivated students with a background in biology, human biology, medicine, veterinary medicine, physics, or related fields who want to work with modern techniques and unusual model organisms in their Bachelor's, Master's, or Ph.D. thesis.
Molecular Imaging

Prof. Dr.
Marcel Lauterbach
| +49 6841/16-16410 |
 |
Curriculum Vitae |
Saarland University
Medical Faculty
Prof. Dr. Marcel Lauterbach
CIPMM | Building 48
D-66421 Homburg (Saar)


